

Note how interpreting the diagram above flows naturally into a discussion about orbitals. The Graph Is Explained By The Periodic Filling of Electronic Energy Levels (aka “Orbitals”) Second, note the periodic trend: there are certain elements (He, Ne, Ar, Kr, Xe) which have unusually high ionization energies, followed by elements (Li, Na, K, Rb, and Cs) with unusually low ionization energies.Ģ.Indeed that “planetary” analogy was the basis for the Bohr model of the atom. We can draw an analogy here to planets orbiting the sun: all else being equal, the further away a planet (electron) is from the sun (nucleus), the less attractive force there will be between the two (as measured by Newton’s law of gravitation in one case, and Coulomb’s law in the other). First, note the broad trend: generally, as elements increase in size, the amount of energy required to pull an electron away from the atom decreases.There is a tremendous amount of information about atomic structure embedded in this extremely simple plot, but the concept itself isn’t so hard to understand: “how much energy does it take to rip an electron off a neutral element?” In other words: “how easy is it to pull off an electron off each element?”. The y-axis shows the amount of energy required to ionize each neutral element to a charge of +1 (the “first ionization energy”).The x-axis shows every element of the periodic table, in increasing order of atomic number.That caveat aside, if one had to name a single graph which could be saved from a cataclysm for the next generation of creatures, my vote would be for this one: You can always trust a barber to find an uplifting quote about the importance of haircuts. Well of course a chemist like myself is going to love a quote that extolls the central importance of chemistry to scientific knowledge.
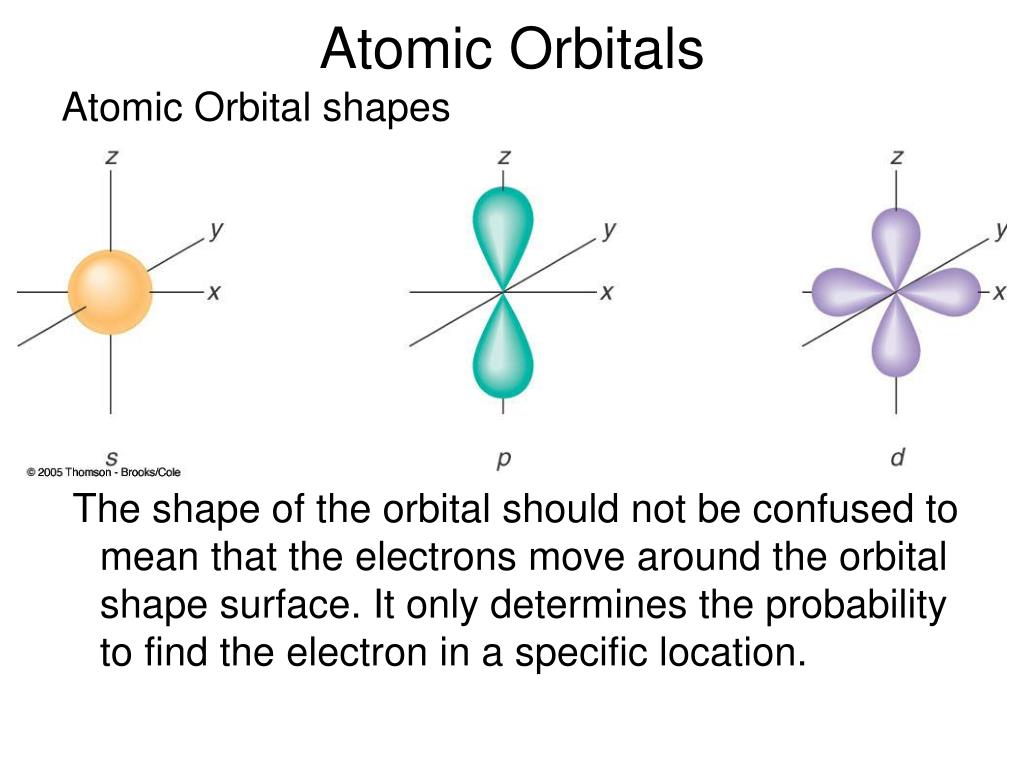
“If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generation of creatures, what statement would contain the most information in the fewest words? I believe it is the atomic hypothesis that all things are made of atoms - little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another. In that one sentence, you will see, there is an enormous amount of information about the world, if just a little imagination and thinking are applied.” The Electronic Configuration Of Fluorine.The 2p Shell: Boron, Carbon, and Nitrogen.The 2s Shell: Electronic Configuration Of Lithium and Beryllium.The 1s shell: Electronic Configuration Of Hydrogen and Helium.A Tour Of The Electronic Configurations of The First 11 Elements.Orbitals Are Defined By The Three Quantum Numbers n, l, and m.The Graph Is Explained By The Periodic Filling of Electronic Energy Levels (aka “Orbitals”).General Chemistry Review: Atomic Orbitals for Organic Chemistry


 0 kommentar(er)
0 kommentar(er)
